Surprise! President Trump signed 4 government orders (EO) final Friday afternoon. They cowl:
- Pass-through of 340B reductions for insulin and EpiPens
- Most Favored Nation (MFN) pricing for medication
The fourth order on MFN has not been publicly launched, so I’ll reserve remark for now.
I see important challenges in implementing these far-reaching orders. Below, you’ll discover the textual content of the primary three together with my commentary and hyperlinks to Drug Channels articles that may show you how to make sense of them.
Read on and see for those who agree with me.
PASS THROUGH OF 340B DISCOUNTS
Text: Executive Order on Access to Affordable Life-saving Medications
The administration needs to mandate that 340B reductions for insulin and EpiPens be handed on to sufferers. Currently, 340B coated entities should purchase these merchandise for $0.01 (penny pricing). The EO due to this fact goals to remove the unfair hidden subsidies that I mannequin in How Hospitals and PBMs Profit—and Patients Lose—From 340B Contract Pharmacies. (Check out the full of life dialogue within the feedback beneath that article.)
However, the EO applies solely to the roughly 1,000 Federally Qualified Health Centers (FQHCs) within the 340B program. That’s stunning, as a result of I usually think about these coated entities to be the great guys of 340B. FQHCs account for lower than 2% of the 112,000 relationships between covered entities and contract pharmacies. Notably, the chief order doesn’t point out hospitals, that are the worst abusers of the 340B program.
The government order can be restricted to “individuals with low incomes,” who’re outlined as having both:
- a excessive price sharing requirement for both insulin or injectable epinephrine;
- or no well being care insurance coverage.
There are not any particulars on how the federal government will operationalize these {qualifications}.
Some of you might doubt the motivation for a 340B government order. I recommend that you simply revisit Federal Oversight of Compliance at 340B Contract Pharmacies Needs Improvement, a 2018 United States Government Accountability Office (GAO) examine.
The GAO discovered that an astounding share of coated entities—33% of federal grantees and 57% of hospitals—don’t present reductions to low-income, uninsured sufferers. Poor sufferers paid full worth, whereas the coated entities collected a giant low cost. See the darkish blue segments of Figure 9, reproduced beneath. Seriously?!?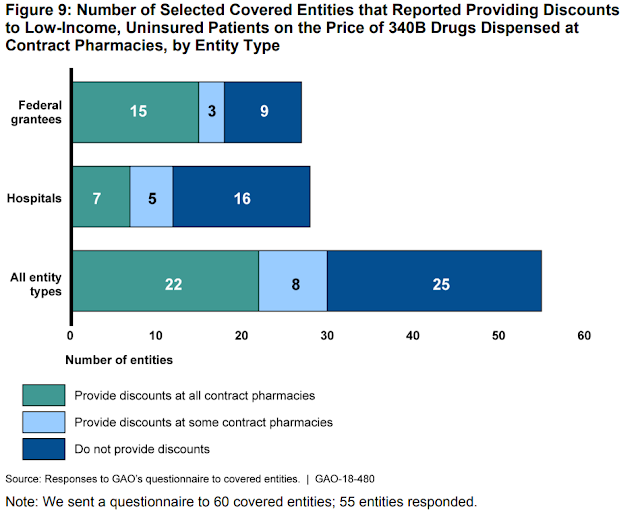
Nonetheless, this government order’s influence will likely be small relative to the entire meting out of insulin and epinephrine. I’m additionally uncertain about whether or not the chief department has the authority to make this variation to the 340B program.
But maybe this can be a helpful first step towards reforming and modernizing it. That’s why you may anticipate great pushback from hospitals, PBMs, and pharmacies. They will all acknowledge the existential risk to their 340B earnings.
REBATE REFORM
Text: Executive Order on Lowering Prices for Patients by Eliminating Kickbacks to Middlemen
As Drug Channels readers know, I’ve lengthy advocated for reforming the rebate system to deal with the warped incentives of the U.S. drug channel. I’ve coated this subject extensively in more than 60 Drug Channels articles with the gross-to-net bubble tag.
I recommend that you simply begin with my ideas on why the rebate rule stalled in 2019: Six Reasons Why the Rebate Rule Failed—And What’s Next.
For now, I stay skeptical that the administration can pull off rebate reform for Medicare Part D given the political and sensible challenges. Here’s what I wrote final August:
“Rebate reform was always going to be a tough sell. It represents a complex, multifaceted change to an opaque system that few people understand. The effects were highly unpredictable and subject to great debate. Few politicians were willing to roll the dice on the administration’s promises that the rebate rule would reduce drug prices.”
These points stay. Also, notice the essential caveat within the textual content of the chief order:
“Prior to taking action under section 3 of this order, the Secretary of Health and Human Services shall confirm — and make public such confirmation — that the action is not projected to increase Federal spending, Medicare beneficiary premiums, or patients’ total out-of-pocket costs.”
To clear these three hurdles, maybe the administration might slim its focus to extremely rebated therapies or specialty medication that deal with few seniors however have very excessive out-of-pocket prices.
Consider hepatitis C therapies, which meet each of those standards. Part D plans typically favor excessive listing worth medication with excessive rebates. These selections elevate the federal authorities’s Medicare spending and needlessly price seniors hundreds in out-of-pocket spending.
The downside: Plans’ rebates from the excessive listing worth merchandise cut back month-to-month premiums for more healthy seniors. This reverse insurance coverage downside is baked into the bizarre math of Part D bidding and its profit construction. For my Part D primer, revisit this text: Why Part D Plans Prefer High List Price Drugs That Raise Costs for Seniors.
Bottom line: I’m protecting a hopeful however skeptical eye on this order.
P.S. For my futuristic imaginative and prescient of how a brand new system might work, you can even revisit A World Without Rebates: Predictions for How the Channel Will Evolve and Why Drug Prices Will Go Down. I can dream, can’t I?
IMPORTATION
Text: Executive Order on Increasing Drug Importation to Lower Prices for American Patients
Like a narcoleptic Jason Voorhees, importation is again … once more!
Last December, the FDA and HHS issued a notice of proposed rulemaking (NPRM) that would permit importation of prescriptions from Canada. The NPRM would enable states and sure different non-federal authorities entities to submit importation program proposals to the FDA for overview and authorization. The HHS claimed that these laws could be comparable to these of the Drug Supply Chain Security Act (DSCSA) and would pose “no additional risk to the public’s health and safety.”
Friday’s government order primarily asks the federal government to end this rulemaking course of.
Unfortunately, importation is a fantasy “solution” that will not present any significant financial savings and may’t work with out trashing the DSCSA track-and-trace legislation. To perceive why, learn my op-ed with Dirk Rodgers: . Our op-ed was written six months before the proposed rule, it remains valid.
HHS and the FDA tried to finesse the track-and-trace realities with a ludicrously convoluted, impractical, and sure unlawful scheme to bypass the DSCSA. And after all, there are numerous different important boundaries to industrial importation, together with opposition from Canada and lack of cooperation from business members.
ICYMI, the FDA released an economic analysis of cost savings from this plan. It concluded: “We are unable to estimate the cost savings from this proposed rule.” And in case anyone missed the snark, the FDA published a blank table titled “Benefits, Costs, and Distributional Effects of Proposed Rule.”
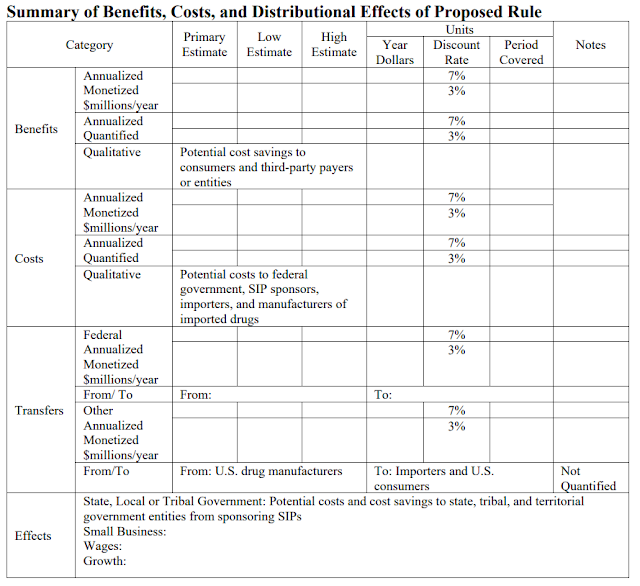
LOL. Can’t make these things up!
What’s extra, I consider that states would lose their Medicaid rebates on medication which can be imported underneath this technique. Say goodbye to paying $0.01 for a lot of brand-name medication! HHS punted on this topic in its proposed rule. None of the state analyses even ponder this subject.
Bottom line: importation is infeasible and never cost-effective. I anticipate it to fail due to the sensible realities of rulemaking and authorized challenges primarily based on the DSCSA.
WHAT’S NEXT
It’s arduous to see any of those government orders having a serious influence earlier than the election. Regardless of the proposals’ particular person deserves, I view them as political theater slightly than implementable, near-term insurance policies. All require substantial further rulemaking and could possibly be topic to numerous authorized challenges.
But if Trump is reelected, anticipate these initiatives to type the spine of our drug pricing discussions over the following few years. After the announcement, Secretary of HHS Alex Azar mentioned:
“So what the President did today is, through executive order, make clear that these are the policies of his administration. These will happen; he has ordered them to happen. Debate is over. They will occur, and so it’s a commission, as with all executive orders across the executive department, to ensure alignment and make sure that we implement according to his directives.”
A daring promise—or the triumph of hope over expertise?